Cerianna™ (fluoroestradiol F18) injection
At a glance
SEEING CLEARER
The only imaging agent that detects whole-body ER+ lesion status
Unique mechanism of action that detects ER+ lesions*†5
Verified concordance with biopsy for determining ER status in MBC* 1
Distinct mode of action that may help inform clinical decisions
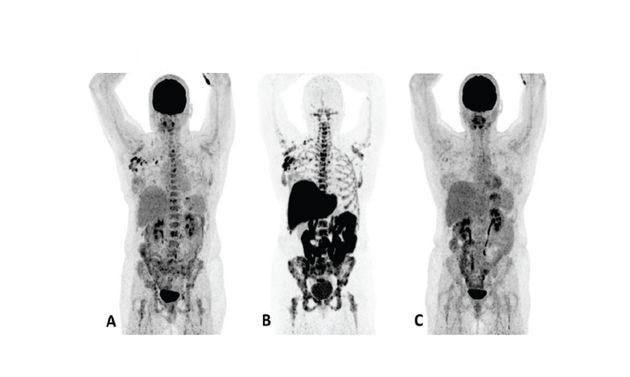
Patient 1 (anterior/superior) responded to treatment with an AI and a CDK 4/6 inhibitor*15
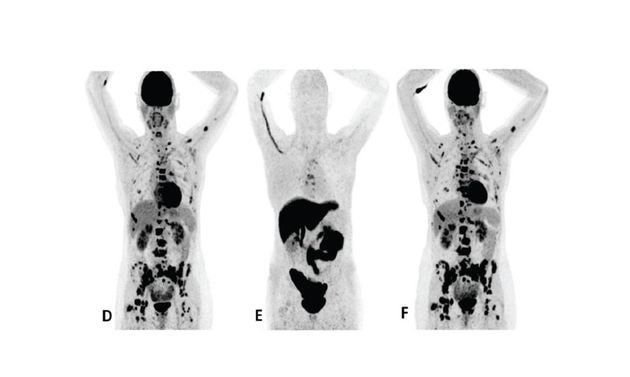
Patient 2 (anterior/superior) did not respond to treatment with an AI and a CDK 4/6 inhibitor*15
*AI = aromatase inhibitor, CDK = cyclin-dependent kinase, FDG = 18F-fluorodeoxyglucose, Tx = treatment.
†Upper row responder: Baseline FDG PET (A) showed pathological uptake in axillary lymph nodes (right side) and in nearly all vertebrae and pelvic bones. Image B showed the baseline F18 fluoroestradiol PET with pathological ER expression in the axial skeleton (including vertebrae, pelvic bones, proximal humeri and femora) and in axillary lymph nodes (right side). After 8 weeks, the FDG PET (C) showed almost complete metabolic response (just some slightly elevated uptake in the axillary lymph nodes). The patient had been on treatment for more than 70 weeks. Lower row non-responder: Baseline FDG PET (D) showed pathological uptake in multiple skeletal lesions. Image E showed the baseline F18 fluoroestradiol PET with only some increased ER expression in thoracic vertebrae. After 8 weeks, the FDG PET (F) showed no metabolic response, even some increase in the pathologic uptake in the multiple skeletal lesions.15
Images from a prospective, single center, feasibility study that aimed to explore whether baseline F18 fluoroestradiol PET discordance and F18 fluoroestradiol uptake were correlated with outcome and response to concomitant treatment with an AI and a CDK 4/6 inhibitor. Thirty patients with ER+ MBC were included in the study, including 87% who received at least one previous line of ET in the metastatic setting.15
Cerianna production site locations
Case Studies
Interpreter Training
Safety
REFERENCES
- 1. Kurland BF, Wiggins JR, Coche A, Fontan C, Bouvet Y, Webner P, et al. Whole-body characterization of estrogen receptor status in metastatic breast cancer with 16α-18F-fluoro-17β-estradiol positron emission tomography: meta-analysis and recommendations for integration into clinical applications. Oncologist. 2020;25(10):835-844.
- 2. Yang Z, Sun Y, Zhang Y, Xue J, Wang M, Shi W, et al. Can fluorine-18 fluoroestradiol positron emission tomography-computed tomography demonstrate the heterogeneity of breast cancer in vivo? Clin Breast Cancer. 2013;13(5):359-363.
- 3. van Kruchten M, Glaudemans AW, de Vries EF, Beets-Tan RG, Schröder CP, Dierckx RA, et al. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med. 2012;53(2):182-190.
- 4. van Kruchten M, de Vries EGE, Brown M, de Vries EFJ, Glaudemans AWJM, Dierckx RAJO, et al. PET imaging of estrogen receptors in patients with breast cancer. Lancet Oncol. 2013;14(11):e465-e475.
- 5. Cerianna (fluoroestradiol F 18) Injection Prescribing Information. Zionexa US Corp
- 6. Kurland BF, Peterson LM, Lee JH, Linden HM, Schubert EK, Dunnwald LK, et al. Between-patient and within-patient (site-to-site) variability in estrogen receptor binding, measured in vivo by 18F-fluoroestradiol PET. J Nucl Med. 2011;52(10):1541-1549
- 7. Currin E, Peterson LM, Schubert EK, Link JM, Krohn KA, Livingston RB, et al. Temporal heterogeneity of estrogen receptor expression in bone-dominant breast cancer: 18F-fluoroestradiol PET imaging shows return of ER expression. J Natl Compr Canc Netw. 2016;14(2):144-147
- 8. Chae SY, Ahn SH, Kim S-B, Han S, Lee SH, Oh SJ, et al. Diagnostic accuracy and safety of 16α-[18F]fluoro-17β-oestradiol PET-CT for the assessment of oestrogen receptor status in recurrent or metastatic lesions in patients with breast cancer: a prospective cohort study. Lancet Oncol. 2019;20(4):546-555
- 9. Amir E, Miller N, Geddie W, Freedman O, Kassam F, Simmons C, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;30(6):587-592.
- 10. Sundararajan L, Linden HM, Link JM, Krohn KA, Mankoff DA. 18F-fluoroestradiol. Semin Nucl Med. 2007;37(6):470-476
- 11. Linden HM, Stekhova SA, Link JM, Gralow JR, Livingston RB, Ellis GK, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24(18):2793-2799
- 12. Cardoso F, Fallowfield L, Costa A, Castiglione M, Senkus E, ESMO Guideline Working Group. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22 Suppl 6:vi25-30
- 13. Linden HM, Peterson LM, Fowler AM. Clinical potential of estrogen and progesterone receptor imaging. PET Clin. 2018;13(3):415-422
- 14. Kurland BF, Peterson LM, Lee JH, Schubert EK, Currin ER, Link JM, et al. Estrogen receptor binding (18F-FES PET) and glycolytic activity (18F-FDG PET) predict progression-free survival on endocrine therapy in patients with ER+ breast cancer. Clin Cancer Res. 2017;23(2):407-415
- 15. Boers J, Venema CM, de Vries EFJ, Glaudemans AWJM, Kwee TC, Schuuring E, et al. Molecular imaging to identify patients with metastatic breast cancer who benefit from endocrine treatment combined with cyclin-dependent kinase inhibition. Eur J Cancer. 2020;126:11-20
- 16. Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19(11):2797-2803
- 17. Boers J, Loudini N, Brunsch CL, Koza SA, de Vries EFJ, Glaudemans AWJM, et al. Value of 18F-FES PET in solving clinical dilemmas in breast cancer patients: a retrospective study. J Nucl Med. 2021;62(9):1214-1220
- 18. Ulaner GA, Jhaveri K, Chandarlapaty S, Hatzoglou V, Riedl CC, Lewis JS, et al. Head-to-head evaluation of 18F-FES and 18F-FDG PET/ CT in metastatic invasive lobular breast cancer. J Nucl Med. 2021;62(3):326-331
- 19. Venema C, de Vries E, Glaudemans A, Poppema B, Hospers, G, Schröder C. 18F-FES PET has added value in staging and therapy decision making in patients with disseminated lobular breast cancer. Clin Nucl Med. 2017;42(8):612-614
- 20. Linden HM, Kurland BF, Peterson LM, Schubert EK, Gralow JR, Specht JM, et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res. 2011;17(14):4799-4805
Important Safety Information
INDICATIONS AND USAGE:
CERIANNA is indicated for use with positron emission tomography (PET) imaging for the detection of estrogen receptor (ER)-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic breast cancer.
Limitations of Use:
Tissue biopsy should be used to confirm recurrence of breast cancer and to verify ER status by pathology. CERIANNA is not useful for imaging other receptors, such as human epidermal growth factor receptor 2 (HER2) and the progesterone receptor (PR).
Important Safety Information
CONTRAINDICATIONS
• None.
WARNINGS AND PRECAUTIONS
Risk of Misdiagnosis
Inadequate Tumor Characterization and Other ER-Positive Pathology
• Breast cancer may be heterogeneous within patients and across time. CERIANNA images ER and is not useful for imaging other receptors such as HER2 and PR. The uptake of fluoroestradiol F 18 is not specific for breast cancer and may occur in a variety of ER-positive tumors that arise outside of the breast, including from the uterus and ovaries. Do not use CERIANNA in lieu of biopsy when biopsy is indicated in patients with recurrent or metastatic breast cancer.
False Negative CERIANNA Scan
• A negative CERIANNA scan does not rule out ER-positive breast cancer. Pathology or clinical characteristics that suggest a patient may benefit from systemic hormone therapy should take precedence over a discordant negative CERIANNA scan.
Radiation Risks
• Diagnostic radiopharmaceuticals, including CERIANNA, expose patients to radiation. Radiation exposure is associated with a dose-dependent increased risk of cancer. Ensure safe drug handling and patient preparation procedures (including adequate hydration and voiding) to protect patients and
health care providers from unintentional radiation exposure.
Pregnancy Status
• Assessment of pregnancy status is recommended in females of reproductive potential before administering CERIANNA.
ADVERSE REACTIONS
• In Clinical Trials (n=1207) the most common adverse reactions seen occurred at a rate < 1% were injection-site pain and dysgeusia.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
• All radiopharmaceuticals, including CERIANNA, have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of radiation dose. Advise a pregnant woman of the potential risks of fetal exposure to radiation from administration of CERIANNA.
• There are no available data on CERIANNA use in pregnant women. No animal reproduction studies using fluoroestradiol F 18 have been conducted to evaluate its effect on female reproduction and embryo-fetal development.
• The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Lactation
Risk Summary
• There are no data on the presence of fluoroestradiol F 18 in human milk, or its effects on the breastfed infant or milk production. Lactation studies have not been conducted in animals. Advise a lactating woman to avoid breastfeeding for 4 hours after CERIANNA administration in order to minimize radiation exposure to a breastfed infant.
Pediatric Use
• The safety and effectiveness of CERIANNA in pediatric patients have not been established.
Geriatric Use
- Clinical studies of fluoroestradiol F 18 injection did not reveal any difference in pharmacokinetics or biodistribution in patients aged 65 and over.
DRUG INTERACTIONS
Systemic Endocrine Therapies that Bind to ER
- Drugs that bind to the ER, including SERMs and SERDs, may compete with the binding of fluoroestradiol F18 and may reduce detection of ER-positive lesions with CERIANNA.
- Before administration of CERIANNA, discontinue drugs that bind to the ER, such as SERMs and SERDs, for at least 5 biological half-lives: ( e.g. elacestrant for 11 days, tamoxifen for 8 weeks and fulvestrant for 28 weeks).
To report SUSPECTED ADVERSE REACTIONS, contact GE HealthCare at 800 654 0118 (option 2 then option 1) or by email at GPV.drugsafety@gehealthcare.com or FDA at 800 FDA 1088 or www.fda.gov/medwatch
