Top image: Peter Arduini, President & CEO of GE HealthCare; Roland Rott, President & CEO of Imaging; Philip Rackliffe, President & CEO of Advanced Visualization Solutions; and Catherine Estrampes, President & CEO of US and Canada
Since the nineties, the pink ribbon has earned its spot as the most recognizable symbol of support for breast cancer awareness and hope for the future globally. In a vibrant and bold display of commitment, four GE HealthCare CEOs are raising eyebrows and funds by unexpectedly popping up at events, meetings and on social media wearing bold pink wigs to show their support for ending breast cancer.
Breast cancer remains the most common cancer in women worldwide and the most commonly diagnosed in women in the United States, with approximately one in eight women facing a diagnosis in their lifetime. This staggering statistic underscores the critical need for early detection and innovative treatment solutions, advanced research, policy initiatives and bold actions.
Since 2016, a handful of GE HealthCare colleagues in Wisconsin have participated in Susan G. Komen’s BigWig competition, raising a total of $122,500.


Philip Rackliffe and Peter Arduini wearing wigs to raise awareness for breast cancer research and improved outcomes
For decades, GE HealthCare has been raising the innovation bar to address breast cancer, beginning with the introduction of mammography 60 years ago, followed by automated breast ultrasound. The company has since advanced radiation therapy guidance using CT, MR, PET imaging - all equipped with AI and optimized for use with diagnostic agents, which the company also develops.
Since its spinoff in 2023, the company has deepened its focus on oncology and improving the patient journey along care pathways. For breast cancer, GE HealthCare enables personalized care at every step of a patient’s unique journey while also raising awareness about the importance of advanced technology, including supplemental screening, and the importance of early detection to save lives.
A commitment to community and advocacy
GE HealthCare's longstanding collaboration with Susan G. Komen extends beyond the BigWig competition.
Together, the two organizations have worked tirelessly to improve breast cancer care through awareness campaigns, policy advocacy, and educational programs like the Metastatic Breast Cancer (MBC) Impact Series, which provides critical resources for the MBC community.
GE HealthCare’s Cerianna™ (fluoroestradiol F 18), a diagnostic imaging tool, reflects the potential binding ability of estradiol in ER+ lesions to equip oncologists to make personalized treatment plans for their patients.
Kimberly Reinika, an actor and event planner with Disney and longtime volunteer with Susan G. Komen, was diagnosed with breast cancer in 2020. Her oncologist included Cerianna in her workup when designing her treatment plan. The information from the scan, biopsy, and several other factors contributed to her overall clinical picture, ultimately resulting in a change in management recommendations from chemotherapy intravenously to taking two pills a day to treat her ER+ breast breast cancer.
Kimberly’s treatment changed from her expecting to receive chemotherapy intravenously to taking two pills a day for her ER- targeted therapy following information from her Cerianna scan, biopsy, and multiple other factors
GE HealthCare teams also work closely with Susan G. Komen on shaping the United States’ national healthcare policy, including advocating for a nationwide reporting standard—that went into effect in 2024—requiring mammography facilities to provide information to patients regarding the density of their breasts.
Additional policy change efforts have included improving access, affordability, and outcomes for the breast cancer community, focusing on issues like the cost of breast imaging, access to treatments and addressing health disparities.
A legacy of continued innovation and evolution in breast cancer care
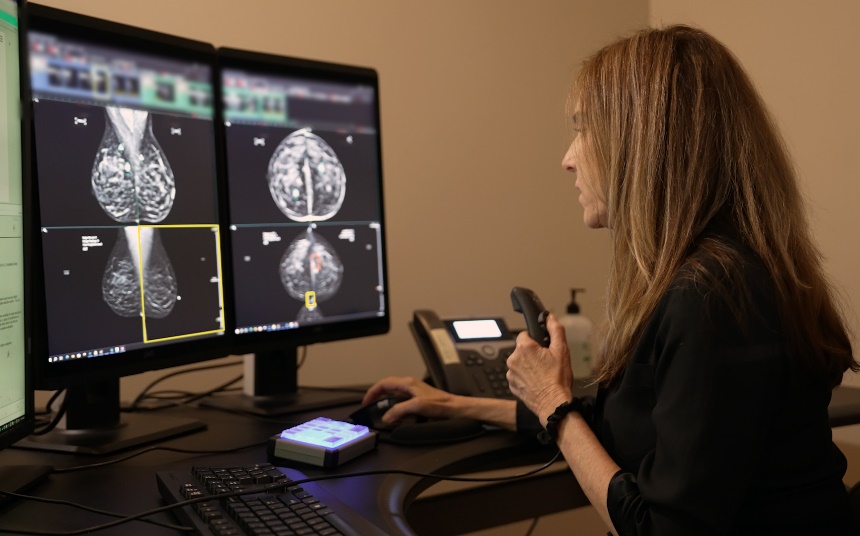
Since its debut, mammography - still considered the gold standard in breast cancer screening - has evolved significantly. From the introduction of digital mammography systems to the development of 3D imaging, AI-enhanced diagnostics, and contrast-enhanced mammography (CEM) and biopsy, GE HealthCare continually pushes the boundaries of what is possible in breast cancer screening and diagnosis.
Digital mammography systems provide higher quality images compared to traditional film mammography, allowing for better visualization of breast tissue, especially in women with dense breasts. The advent of 3D imaging further improved detection performance offering clearer and more detailed images, while AI integration can help enhance diagnostic accuracy by helping radiologists identify potential areas of concern.
CEM represents another leap forward, using a contrast agent to highlight areas of abnormal blood flow in the breast. This technique is particularly useful for detecting cancers that may not be visible on standard mammograms, offering a critical tool for early detection.
“Having watched close friends and family members battle breast cancer, I have seen firsthand the toll it can take on someone,” said Catherine Estrampes. “I have also seen firsthand the impact of technology in the hands of healthcare providers to benefit patients. As a leader in breast health screening and diagnostic technology, GE HealthCare is committed to advancing women’s health and care that can empower personalized breast cancer care for all patients.”
Catherine Estrampes and Roland Rott wigging out with their pets
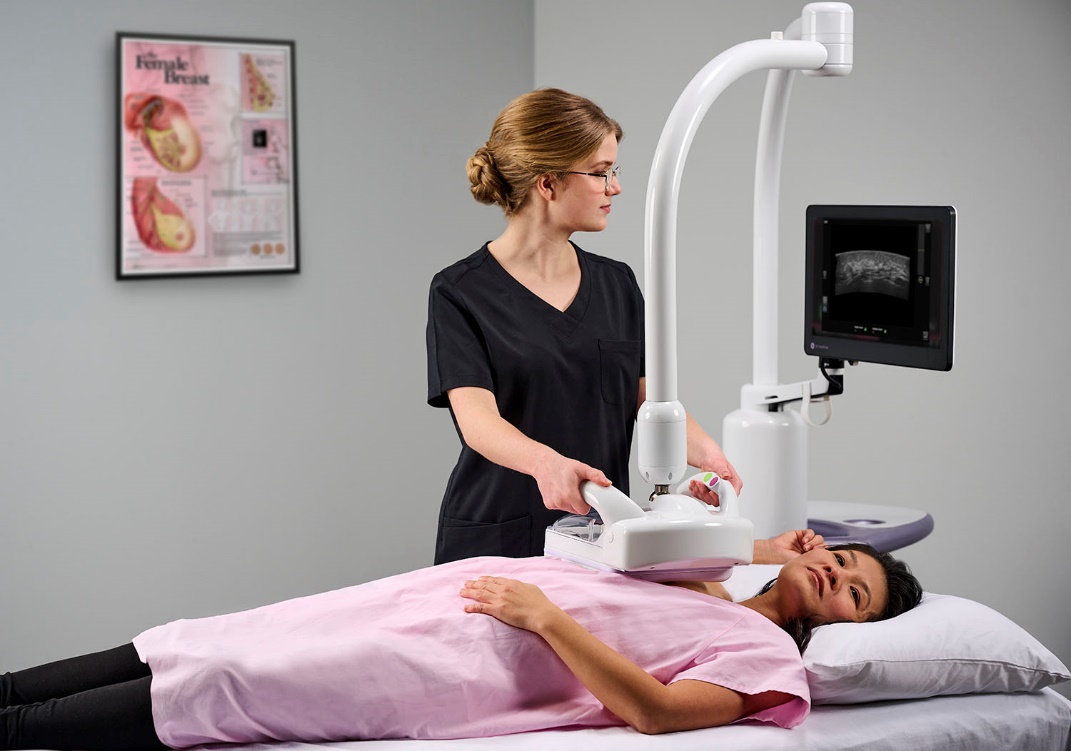
To enhance the patient experience, GE HealthCare introduced Pristina Dueta™, the industry’s first patient-assisted compression device in mammography, allowing women to actively participate in determining their level of breast compression using a hand-held remote control.
As GE HealthCare continues to evolve with an industry facing staffing shortages and rising incidence rates, the company launched the Pristina Via™[1] mammography system to streamline workflow and humanize care. The system, which can be used with Pristina Dueta, continues to shape the future of mammography by providing technologists sophisticated tools that balance the demands of diagnostic accuracy and fast-paced workflows to facilitate more patient-centered breast care.
The Senographe, introduced in 1966, was the first commercially available breast-dedicated X-ray imaging equipment.
Pristina Via mammography system is designed to enhance the screening experience for both technologists and patients.
Most recently, the company launched Invenia™ Automated Breast Ultrasound (ABUS) Premium, the latest 3D ultrasound offering featuring advanced AI and innovative capabilities to drive faster[2], reproducible screening and streamline exam readings for patients with dense breasts. This technology is designed to ensure that patients receive accurate and timely diagnoses, critical for effective treatment planning.
Invenia ABUS Premium features new integrated Verisound AI and AI Assistant, which ensure faster and reproducible scanning and reading with extraordinary image quality.
In addition to its own innovations, GE HealthCare collaborates with organizations including DeepHealth, a global leader in AI-powered health informatics, to address key challenges across the imaging value chain, improving speed, operational efficiency, and elevating patient care. The collaboration combines DeepHealth’s AI-powered SmartMammo™[3] solution and GE HealthCare’s Senographe Pristina™ mammography system to enhance breast care.
The collaboration leverages GE HealthCare’s legacy and scale of imaging innovation and DeepHealth's AI-powered clinical and workflow solutions to further the innovation, and adoption of AI in imaging, while enhancing the way women receive breast care.
Looking ahead
GE HealthCare's commitment to breast cancer care is unwavering. The company continues to innovate to improve patient outcomes and deliver personalized care by empowering clinicians and patients with tools for early detection, accurate diagnosis, and effective treatment.
As Tanya Carrillo, Global Research Director of Patient Care Solutions at GE HealthCare and breast cancer survivor, notes, “After years of working on research in Breast Imaging at GE HealthCare, I became the patient. This reinforced what I already knew from my work such as the importance of early detection through screening and having a strong medical team to partner with me in navigating my care.”
As critically important as medical technology and digital tools are to accomplishing this mission, technology can only do so much. Through their collaboration with Susan G. Komen, GE HealthCare is not only advancing breast cancer care but also fostering a community of support and advocacy.
These efforts help pave the way for a future where breast cancer is detected early, treated effectively, and ultimately, eradicated.
For more information on the Susan G. Komen BigWig campaign and to donate, click here.
Indications and usage of Cerianna™:
Indications and usage
CERIANNA is indicated for use with positron emission tomography (PET) imaging for the detection of estrogen receptor (ER)-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic breast cancer.
Limitations of use
Tissue biopsy should be used to confirm recurrence of breast cancer and to verify ER status by pathology. CERIANNA is not useful for imaging other receptors, such as human epidermal growth factor receptor 2 (HER2) and the progesterone receptor (PR).
Contraindications
None.
Adverse reactions
In Clinical Trials (n=1207) the most common adverse reactions seen occurred at a rate < 1% were injection-site pain and dysgeusia.
To report SUSPECTED ADVERSE REACTIONS, contact GE HealthCare Company at 800.654.0118 (option 2 then option 1) or by email at GPV.drugsafety@gehealthcare.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For full prescribing information click here.