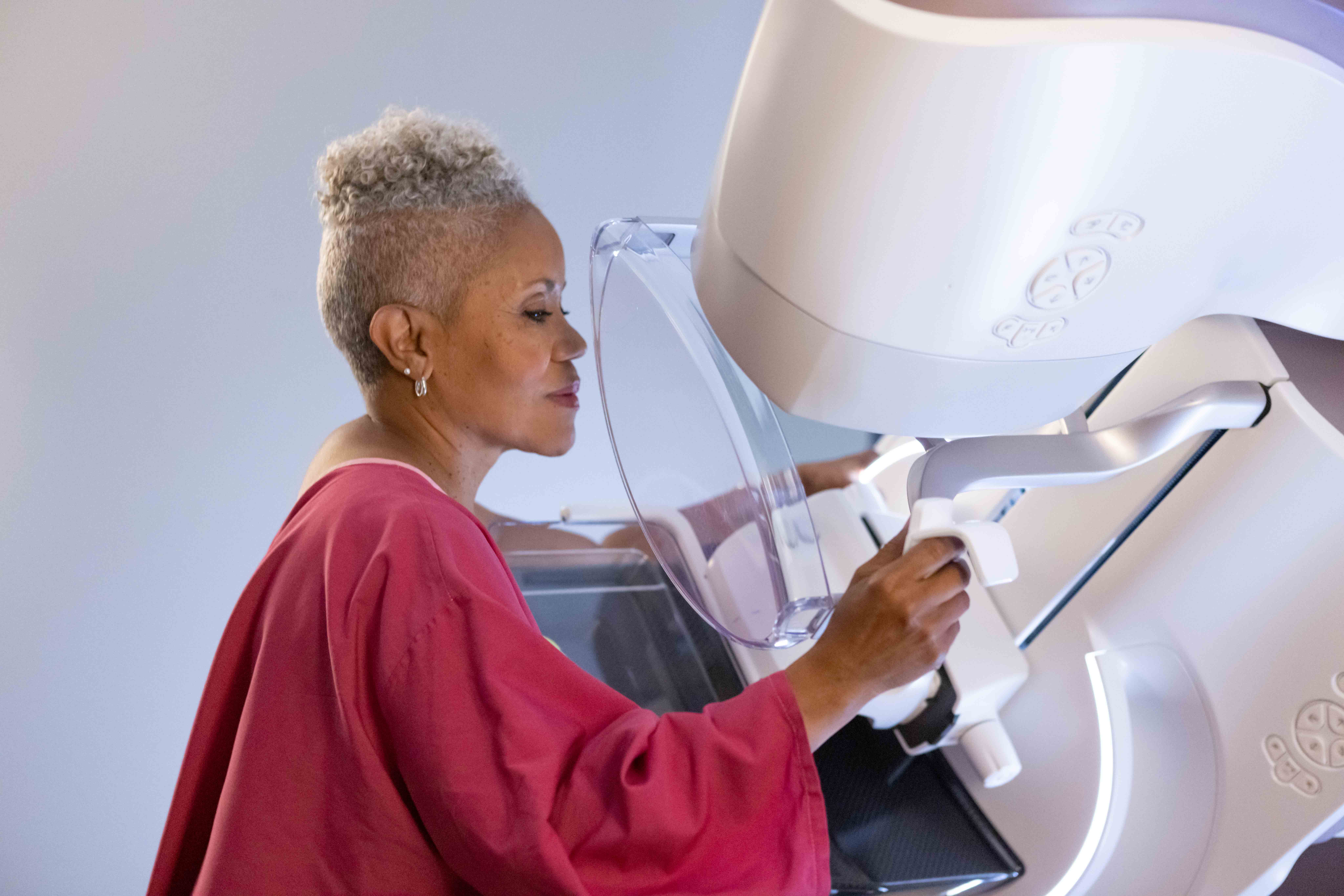
Breast cancer is the most commonly diagnosed cancer among American women, with about one in eight women facing a diagnosis in their lifetime.[1] Regular mammograms are essential to early detection,[2] but another lesser-known benefit of mammograms is that they can also help women determine if they have dense breast tissue.
Unfortunately, many women are unaware of their breast density and do not have a strong understanding of the risk associated with breast density relative to other risk factors.[3]
After years of advocacy and varying policies from state to state, the U.S. Food and Drug Administration announced a new nationwide reporting standard effective September 10, 2024 that requires mammography facilities to provide information to patients regarding the density of their breasts.
The new nationwide reporting standard under the Mammography Quality Standards Act (MQSA) of 1992 ensures all women receive “specific language explaining how breast density can influence the accuracy of mammography.”[4] In addition to requiring notification, the regulations will also require facilities to recommend that patients with dense breasts talk to their healthcare provider about breast density, risks for breast cancer, and their individual situation.
Providing this kind of information not only offers hope for earlier diagnosis but can also help facilitate important conversations between patients and their providers to better understand personal risk factors and supplemental imaging options available to provide more personalized breast care.
As a leader in breast health screening and diagnostic technology, GE HealthCare is committed to advancing women’s health and care that can empower personalized breast cancer care for all patients.
Learn more about the different technologies available to support early detection and personalized breast care for women with dense breasts.
Considering dense breasts in breast cancer screening and detection
Breasts are made of fat and glands held together by fibrous tissue. When the breast has more glands and fibrous tissue, or a higher proportion of fibroglandular tissue than fatty tissue, the “denser” the breast tissue.
Dense breast tissue is considered common but can put women at a four to six times higher risk for developing breast cancer. [5]
While mammography is considered the most reliable breast imaging technique, dense breast tissue can make the detection of cancer more challenging because tissues overlap and mask small lesions.
In order to help spot breast cancer in its earliest stages, regular screening mammograms along with supplemental screening options can improve outcomes for those at risk of or diagnosed with breast cancer.
Optimizing breast care with advanced imaging technologies
To help improve outcomes for people facing this difficult diagnosis, GE HealthCare offers a comprehensive portfolio of breast imaging technologies and diagnostics to help clinicians provide more personalized breast care to a broad and diverse population.
Supplemental screening technologies for women with dense breasts could include:
- 3D mammography in traditional screening mammography is improving breast cancer detection [6] and its adoption has been swift. GE HealthCare’s Senographe Pristina 3D mammography system was designed to provide a better patient experience [7] and optimized for rapid diagnosis, while also delivering superior image quality and diagnostic accuracy at the same low dose as 2D systems.
- Breast ultrasound uses high-frequency sound waves to produce images of the breast tissue. It can detect tumors that may not be visible on a mammogram, especially in women with dense breast tissue. GE HealthCare’s automated breast ultrasound (ABUS) was the first FDA-approved ultrasound system for screening women with dense breast tissue. When used in addition to mammography, Invenia ABUS can improve breast cancer detection by 35.7 percent over mammography alone. [8]
- Breast MRI uses its magnetic field and radio waves to create detailed images of the breast tissue. GE HealthCare’s breast MRI is a highly sensitive imaging tool that can detect small tumors that may not be visible on a mammogram or ultrasound.
- Contrast-enhanced mammography (CEM) is an innovative technology that can be used when a patient’s result remain inconclusive after screening. GE HealthCare’s SenoBright HD combines mammography and iodinated contrast media to highlight areas of unusual blood-flow patterns that may indicate malignancy - helping reduce the masking effect of fibroglandular breast tissue and increase the clarity of lesions.[9] [10] [11]
Learn more about GE HealthCare’s portfolio of solutions designed to provide a comprehensive approach to advancing personalized breast cancer care for all patients, including women with dense breast tissue at https://www.gehealthcare.com/breastcare.
Disclaimer: Not all products or features are available in all geographies. Check with your local GE HealthCare representative for availability in your country.
[1] https://www.breastcancer.org/facts-statistics
[2] https://pubmed.ncbi.nlm.nih.gov/25928287/
[3] Beidler LB, Kressin NR, Wormwood JB, et al. Perceptions of breast cancer risks among women receiving mammograph screening. JAMA Network Open. 2023;6(1):e2252209. doi:10.1001/jamanetworkopen.2022.52209.
[4] U.S. Food and Drug Administration. FDA Updates Mammography Regulations to Require Reporting of Breast Density Information and Enhance Facility Oversight (press release). March 9, 2023. Available at: https://www.fda.gov/news-events/press-announcements/fda-updates-mammography-regulations-require-reporting-breast-density-information-and-enhance. Last accessed March 2023.
[5] https://www.nejm.org/doi/full/10.1056/nejmoa062790
[6] Bernardi D, Macaskill P, Pellegrini M, et al. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): a population-based prospective study. Lancet Oncol. 2016 Aug;17(8):1105-1113. doi: 10.1016/S1470-2045(16)30101-2.
[7] IPSOS Patient Satisfaction Study sponsored by GE HealthCare, conducted with 315 patients across 2 sites in Europe, February 2017
[8] FDA PMA P110006 summary of safety and effectiveness.
[9] Compared to without contrast
[10] Jochelson MS, Lobbes MBI. Contrast-enhanced Mammography: State of the Art. Radiology J. Sung et al., Radiology 2021; 299:36-48
[11] 510(k) K172404