Many drugs are known to cause a long QT interval, including some antidepressants, antipsychotics, antibiotics, opioids, and the co-administration (polypharmacy) of certain medications. In October 2022, for example, the American College of Cardiology warned about potential interactions with the Emergency Use Authorization-granted COVID-19 drug Paxlovid, noting how combining it with the angina therapy ranolazine can prolong QT.1
As Healio notes, because patients with prolonged QT who take these medications are at risk of fatality from torsades de pointes (TdP), many labels indicate that QT intervals should be measured before the therapeutic regimen begins. That way, providers can assess the patient's safety and establish baselines for monitoring future changes.2
But with the rise in remote and digitized care, that's not always possible. Accordingly, the landscape surrounding mobile QT monitoring has advanced a great deal in just the past few years, evidenced by developments such as the FDA granting 510(k) clearance to AliveCor's KardiaMobile 6L for QTc assessments amid increasing acceptance and practicality of home QT measurement.
While this new generation of QT monitoring does present a viable option for assessing QT intervals in some situations outside the clinic, these devices should not be used exclusively. Indeed, multi-lead ECG, and 12-lead in particular, reliably remains the gold standard at the point of care, especially for critical care patients. Here's why.
12-Lead ECG: The Gold Standard
Due to the comprehensive insights available from 12-lead ECG, this method is often preferred for initial and ongoing QT assessment. As the authors write in a 2020 Journal of General Internal Medicine article, that gold standard recommendation is thanks to a computer's ability to take an average reading of all 12 leads for QTc calculations.3 This isn't practical for a human, but a computer can examine all 12 leads simultaneously to find one onset for QRS and one offset for the T-wave.
Notably, 12 leads are better than three or five partly because of the QT variances often seen from lead to lead. Those deltas—known as QT dispersion—can amount to as much as a 60-millisecond difference, which could influence potential care pathways and interventions for critical care patients.3 Consequently, a baseline change of more than 60 milliseconds is stratified as a grade 3 QTc prolongation by the National Cancer Institute, and each 10-ms increase is associated with a 5-7% increase in TdP risk.4
In addition to the potential QT variations between leads, interpreted values themselves may also differ from system to system. For example, one peer-reviewed paper from Cureus reported a study indicating QT intervals might be longer when assessed through continuous telemetry monitoring than by the 12-lead ECG.5 This can also be due to heart rate changes. Ambulatory ECGs achieve higher heart rates. When the QT is corrected for heart rate, it can seem longer than when simply doing ECGs at the same heart rate—this is more likely when performing a 12-lead ECG at rest.
In a critical care patient whose case hangs in the balance between getting lifesaving medications or experiencing fatal TdP, the accuracy of 12 leads makes all the difference. Yet, it's not just about those 12 individual waveforms. Advancements in 12-lead ECG systems offer new capabilities such as threshold breaches that feature automated alerting of QTc risks.
Alerting could preempt tragedies such as the one Dr. Payal Kohli discussed in 2021 where a 29-year-old patient had his QT interval prolonged drip by drip from the anti-arrhythmic amiodarone, which had been given "almost reflexively, without consideration of the effects of amiodarone on the QT interval."
"After this tragic loss, which I am sure will haunt me for decades to come, I have never again underappreciated the power of the ECG in clinical medicine, particularly in cases of QT interval prolongation and torsades de pointes," she wrote.
To learn more about the power of the ECG in today's clinical landscape, browse our Diagnostic ECG Clinical Insights Center.
Strategies for Detecting Long QT Interval with 12-Lead ECGs
Recognizing drug-induced prolonged QT requires a multistep process. The steps are as follows:
- Measure the QT (QRS complex, ST segment, T wave, and only U wave if it's abnormal).
- Measure the RR (peak of QRS complex, ST segment, T wave, U wave, and peak of subsequent QRS).
- Correct for heart rate using one of the many available formulas, such as Bazett: QTc = QT multiplied by (HR/60).1,2 Seeing as all heart correction formulas have limitations, when possible, acquire the ECG during a fixed heart rate between 60 and 100 beats per minute.
- Assess for obvious long QT if QT is greater than half of RR.
- Compare QT with normal sex-based averages (under 470 ms for men and under 480 ms for women).
Seek additional troubleshooting methods if you encounter the following difficulties:
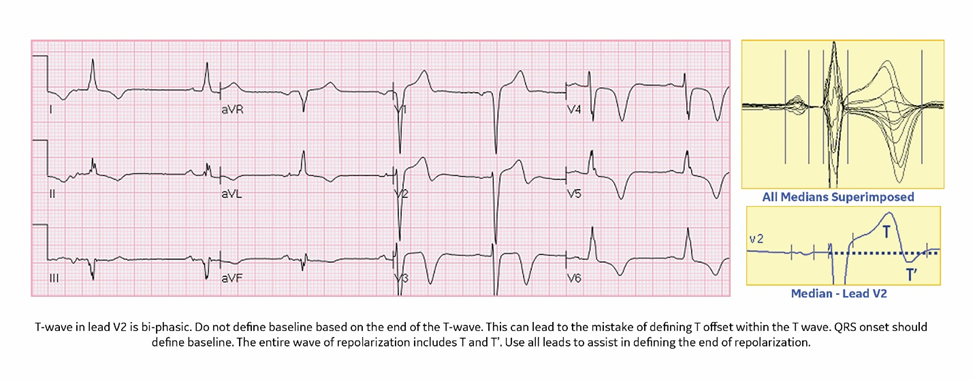
- A biphasic T wave in lead two: Define the baseline by QRS onset, not the end of the T wave. Use all the leads to assist.
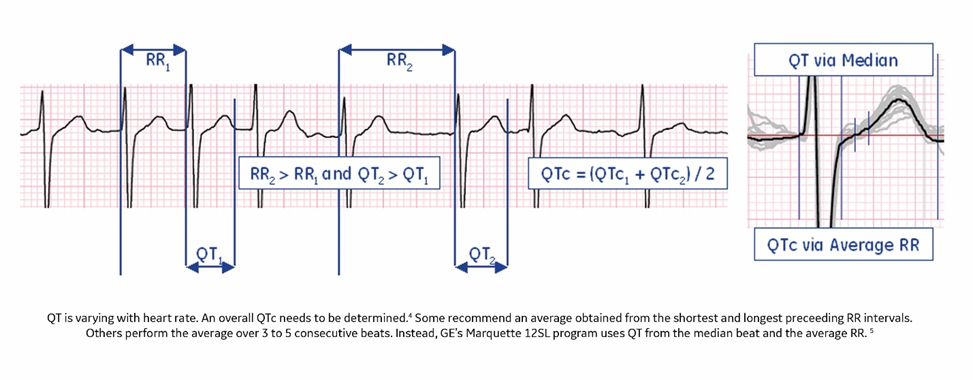
- A varying heart rate: Determine an overall QT interval and RR by employing one of the many available methods. For instance, you could use the QT of the median beat and the average RR.
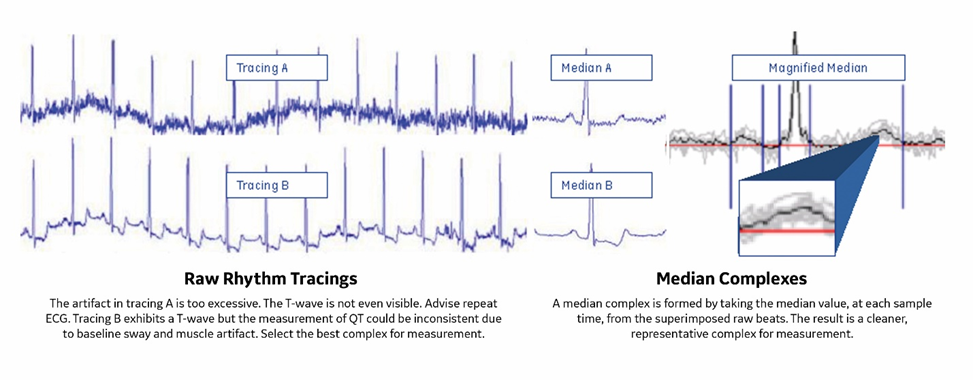
- Excessive artifact tracings: Repeat ECG until a delineated waveform is established, or use median complexes from superimposed beats for a cleaner depiction.
A Must-Have Tool for Accurate QT Monitoring
While alternatives exist for the trusty gold standard of 12 leads—and while single-lead and continuous monitoring devices do serve many ECG use cases, such as identifying arrhythmias—those mobile options may not always be suitable for critical care patients when monitoring for QTc.
After all, providers must have fast and accurate tools available for waveform analysis of QT intervals and potential prolonged QTc, both before drug administration and during therapy. The stakes are significant among critical care populations given the risks of TdP and tragic anecdotes like the one from Dr. Kohli.
For these more vulnerable patients, using 12-lead ECG can help contextualize more nuanced cardiovascular diagnoses such as prolonged QT interval with more leads and, thus, more information.
But remember: The advantages of 12-lead ECG don't just stop at lead quantity. Advanced systems also help surveil status and alert clinical teams to emerging risks. Coupled with the added context and information from 12-lead platforms, these innovations add an intelligent monitoring component atop traditional workups.
Such developments are certainly a step in the right direction: With roughly 12,000 annual cases of drug-induced TdP in the United States, every tool, insight, and lead stands to help combat this serious and potentially deadly complication.6
Resources:
- American College of Cardiology. COVID-19 drug Paxlovid may interact with common cv medications. ACC.org. https://www.acc.org/latest-in-cardiology/articles/2022/10/12/18/19/covid-19-drug-paxlovid-may-interact-with-common-cv-medications. Accessed January 3, 2023.
- Healio. Drug labels, QT prolongation and ECG recommendations. Healio.com. https://www.healio.com/news/cardiology/20191007/drug-labels-qt-prolongation-and-ecg-recommendations. Accessed January 3, 2023.
- Indraratna P, Tardo D, Delves M, et al. Measurement and management of QT interval prolongation for general physicians. Journal of General Internal Medicine. 2019;35(3):865-873. https://doi.org/10.1007/s11606-019-05477-7.
- Kim P, Irizarry-Caro J, Ramesh T, et al. How to diagnose and manage QT prolongation in cancer patients. Journal of the American College of Cardiology CardioOncoloy 2021; 3(1):145–149. https://doi.org/10.1016/j.jaccao.2021.01.002.
- Nasser MF, Jabri A, Kumar A, et al. Lessons learned during the pandemic: Correlation of QT intervals between telemetry and 12-lead electrocardiogram. Cureus. 2021;13(8): e16877. https://www.cureus.com/articles/60408-lessons-learned-during-the-pandemic-correlation-of-qt-intervals-between-telemetry-and-12-lead-electrocardiogram.
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. https://www.ahajournals.org/doi/10.1161/CIR.0000000000000558.