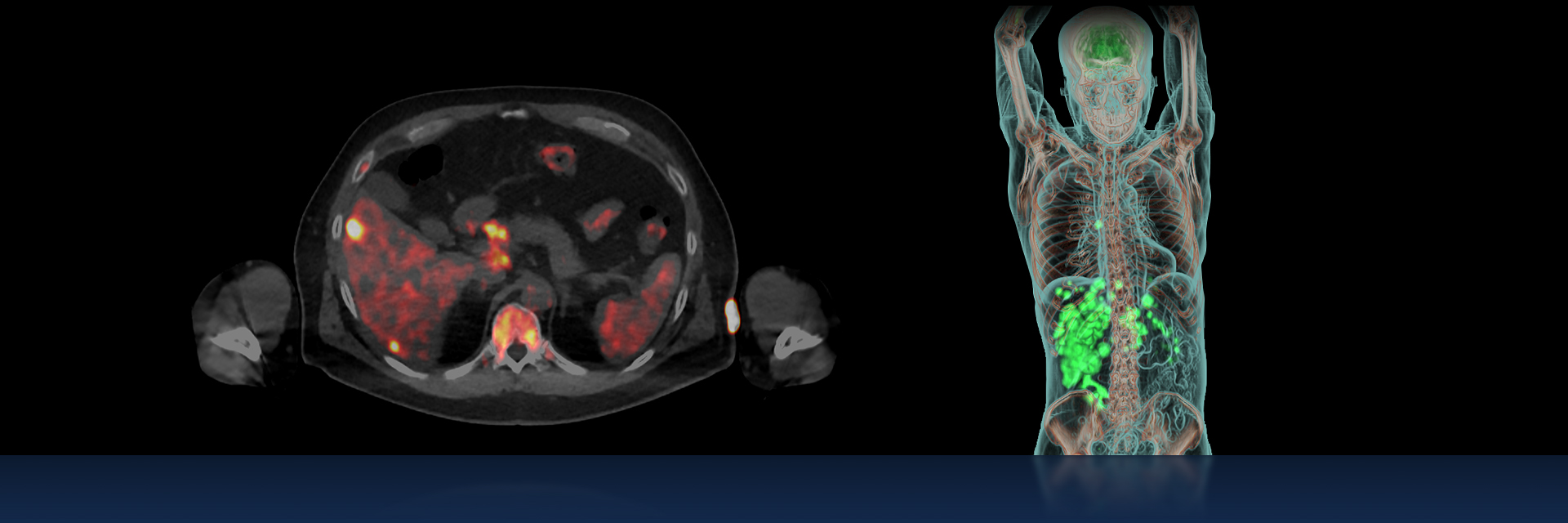
For decades, molecular imaging with single photon emission computed tomography (SPECT) and positron emission tomography (PET), has been a critical imaging tool in oncology, paving the way for metabolic assessment of tumors and quantifying tumor processes. The field of nuclear medicine has rapidly improved diagnostic capabilities in oncology not only because of advances in imaging technologies, but also because of the discovery and development of new tracers, and more recently artificial intelligence (AI)-embedded image processing tools.
As a mature industry, molecular imaging’s next frontier may be more complex, but holds even more potential to uphold the promise of precision medicine and personalized care. The development of novel diagnostic radiopharmaceuticals and targeted therapeutic pairings, working with sophisticated detection technology make advanced diagnosis and targeted treatments possible, reinforcing molecular medicine’s key role in patient management.
Developing new, more specific imaging and therapeutic agents using radiometals
With countless publications validating its significant value to clinicians, nuclear medicine now provides diagnostic, prognostic, predictive and intermediate endpoint biomarkers beyond oncology applications. They extend to cardiology, neurology, and infectious and inflammatory disorders.[1] Using advanced PET imaging and novel imaging biomarkers, whole-body target expression can be quantified and used for optimizing and predicting therapy response in oncology. In pursuit of these and other clinical endeavors, the use of positron-emitting radiometals for PET diagnostic imaging and therapy is increasing.[2]
The use of radiometals supports the continuous development of new, complementary, and more specific biological targeting agents.[3] Gallium-68 (68Ga) has become an option for the radiolabeling of somatostatin analogs, which slow down or stop the body’s production of certain hormones, and prostate-specific membrane antigen (PSMA) ligands for the detection of neuroendocrine and prostate tumors, respectively. The landscape of radiometals used in medical applications is changing rapidly as is the progress in the development, manufacture, and distribution of new radiotracers.
Radiopharmaceuticals labelled with other radionuclides from elements such as Technetium (Tc), Zirconium (Zr), Yttrium (Y), and Copper (Cu) are also frequently used for both diagnostic SPECT and PET imaging applications and radiotherapeutic purposes. As a prominently utilized PET imaging biomarker, radiolabeled fluorodeoxyglucose (18FDG) will continue to be used for many clinical applications, but the short half-life of Fluorine-18 (18F) is not ideal for imaging some biological mechanisms that move more slowly. For certain clinical applications, comprehensive imaging would require radionuclides with a much longer half-life to enable quantifying radioactivity, tracer kinetics and biological uptake beyond few hours after administration.[4]
Radiometal nuclides for diagnostic tracers can be produced by generators and cyclotrons. Generators offer a dose-on-demand convenience but are limited in how much they can produce and need to be replaced on a regular basis. Cyclotron production can begin the process in a solid or liquid state. Irradiation of liquid targets have received growing attention due to compatibility with commonly available medical cyclotrons, a convenient and cost-effective production method of radionuclides 5, A, B. Solid targets have been traditionally viewed as research tools and have required complicated infrastructure and highly trained operators. Recent advancements in solid target technology are paving an alternative pathway for a simple and reliable supply of radionuclides to help satisfy the growing demand for radiometal-based tracers C.
Theranostics offers new options in prostate cancer treatment
Prostate cancer is the second most commonly diagnosed cancer among men worldwide.[5] Traditional imaging techniques for prostate applications utilized 18F or Choline C-11 (11C), however, these biomarkers, when used on patients with prostate specific antigen (PSA) levels under 2nc/mL often yielded poorer detection rates than in those patients with higher PSA levels.[6] This motivated the development of more specific probes in this clinical area.
Because molecular imaging provides ample opportunity to map numerous physiological or pathophysiological targeted processes at the whole-body level, the integration of molecular imaging with radionuclide therapy, also known as theranostics, has paved the way towards highly sensitive radionuclide-based precision medicine. Using a radiolabeled target for both imaging and therapy in prostate cancer has had great success. Radiohybrids, which can be used for both imaging and therapy, encompass an innovative and efficient labeling method to facilitate broad application.[7]
The widely used radionuclide therapy application of choice is Lutetium-177 (177Lu).[8] Its clinical applications have found specific success in both prostate cancer treatment as well as in treating neuroendocrine tumors.[9] 177Lu-dotatate, a peptide receptor radionuclide therapy, was shown to be more effective than high-dose long-acting-release octreotide, commonly used in midgut neuroendocrine tumors, which led to its regulatory approval.[10] Delivering radiation therapy directly to prostate cancer cells in a recent clinical trial, another newly developed lutetium-based therapy called 177Lu-PSMA-617* showed that along with other standard treatments, prostate cancer patients lived longer than those who received only standard therapies: a median of 15.3 months versus 11.3 months.[11]
Recognizing the importance of industry standardization and guidelines for future success
As more new radiolabeled markers are developed, the importance of standardizing production and creating guidelines for use becomes critical to eliminate variations that would impede the ability for treatments to be routinely effective. When 18FDG was initially introduced and produced in academic environments for evaluation, industry leaders recognized that for it to become ubiquitous, it required industry standardization and creation of international usage guidelines.
Variations could be caused by a number of factors including the patient’s physiological condition, various drug intakes, different fasting procedures, and delay times between injection and acquisition. For these reasons, the European Association of Nuclear Medicine (EANM) launched the EANM Research GmbH (EARL) initiative in 2006 to promote the standardization of PET practices and control the many technical and physical factors to make an optimized and accurate link between the patient and PET imaging data. Since then, numerous international guidelines have been published to standardize these procedures.[12]
Similarly, the introduction of new radiolabeled tracers will need the same type of scrutiny in terms of guidelines for production and use. Experts agree that because of the wide application potential of some targets, new approval protocols should be evaluated to expedite their clinical use and potential to improve outcomes.[13] As innovation continues in molecular imaging, the need for knowledgeable clinicians, radiologists, and physicists is essential to maximize the potential that lies ahead, as well as commercial producers who can scale production and bring these new biomarkers to assist with improved patient outcomes.
Learn how GE Healthcare is innovating at each step from discovery to treatment, driving the future of molecular imaging to be more personal.
* Radiopharmaceuticals may not be approved by ministers of health in all regions. 177Lu-PSMA is not an FDA approved tracer.
Not all products or features are available in all geographies. Check with your local GE Healthcare representative for availability in your country.
References
Recent publications using GE equipment supporting liquid target:
- Gagnon et. al. Cyclotron-based 68Ga production with dual liquid targets. Eur J Nucl Med Mol Imaging (2020) 47 (Suppl 1): S1–S753, OP-954
- Rodnick, M.E., Sollert, C., Stark, D. et al. Cyclotron-based production of 68Ga, [68Ga]GaCl3, and [68Ga]Ga-PSMA-11 from a liquid target. EJNMMI radiopharm. chem. 5, 25 (2020)
Reference for GE’s efforts in making solid target easy and accessible:
- J. Svedjehed, M. Pärnaste and K. Gagnon, Demystifying solid targets: Simple and rapid distribution-scale production of [68Ga]GaCl3 and [68Ga]Ga-PSMA-11, Nuclear Medicine and Biology, https://doi.org/10.1016/j.nucmedbio.2021.10.002
[1] Weber WA, Czernin J, Anderson CJ, Badawi RD, Barthel H, Bengel F, Bodei L, Buvat I, DiCarli M, Graham MM, Grimm J, Herrmann K, Kostakoglu L, Lewis JS, Mankoff DA, Peterson TE, Schelbert H, Schöder H, Siegel BA, Strauss HW. The Future of Nuclear Medicine, Molecular Imaging, and Theranostics. J Nucl Med. 2020 Dec;61(Suppl 2):263S-272S. doi: 10.2967/jnumed.120.254532. PMID: 33293447.
[2] An Overview of PET Radiochemistry, Part 2: Radiometals
Marie Brandt, Jens Cardinale, Margaret L. Aulsebrook, Gilles Gasser, Thomas L. Mindt
Journal of Nuclear Medicine Oct 2018, 59 (10) 1500-1506; DOI: 10.2967/jnumed.117.190801
[3] do Carmo, S.J.C., Scott, P.J.H. & Alves, F. Production of radiometals in liquid targets. EJNMMI radiopharm. chem. 5, 2 (2020). https://doi.org/10.1186/s41181-019-0088-x
[4] do Carmo, S.J.C., Scott, P.J.H. & Alves, F. Production of radiometals in liquid targets. EJNMMI radiopharm. chem. 5, 2 (2020). https://doi.org/10.1186/s41181-019-0088-x
[5] https://www.wcrf.org/dietandcancer/prostate-cancer-statistics/
[6] Duclos, V.; Iep, A.; Gomez, L.; Goldfarb, L.; Besson, F.L. PET Molecular Imaging: A Holistic Review of Current Practice and Emerging Perspectives for Diagnosis, Therapeutic Evaluation and Prognosis in Clinical Oncology. Int. J. Mol. Sci. 2021, 22, 4159. https:// doi.org/10.3390/ijms22084159
[7] https://www.snmmi.org/NewsPublications/NewsDetail.aspx?ItemNumber=32003
[8] Das T, Banerjee S. Theranostic Applications of Lutetium-177 in Radionuclide Therapy. Curr Radiopharm. 2016;9(1):94-101. doi: 10.2174/1874471008666150313114644. PMID: 25771364.
[9] Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-DOTATATE for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135.
[10] Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135.
[11] https://www.cancer.gov/news-events/cancer-currents-blog/2021/prostate-cancer-psma-radiopharmaceutical-vision
[12] Aide, N.; Lasnon, C.; Veit-Haibach, P.; Sera, T.; Sattler, B.; Boellaard, R. EANM/EARL harmonization strategies in PET quantification: From daily practice to multicentre oncological studies. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 17–31
[13] Weber WA, Czernin J, Anderson CJ, Badawi RD, Barthel H, Bengel F, Bodei L, Buvat I, DiCarli M, Graham MM, Grimm J, Herrmann K, Kostakoglu L, Lewis JS, Mankoff DA, Peterson TE, Schelbert H, Schöder H, Siegel BA, Strauss HW. The Future of Nuclear Medicine, Molecular Imaging, and Theranostics. J Nucl Med. 2020 Dec;61(Suppl 2):263S-272S. doi: 10.2967/jnumed.120.254532. PMID: 33293447.